Attending the AHA 2023 scientific sessions last weekend at the Philadelphia convention center, Philadelphia, Pennsylvania provided attendees with insight into the ongoing developments throughout the world in the battle to treat cardiovascular disease. With the development of novel technologies one thing is clear: the battlefronts may be changing. In the near future, we may be addressing cardiovascular disease with new classes of medications, and using novel tools for the early identification and treatment of heart disease. It is impossible to keep track of all the innovative science, however we will review some of the most pertinent trials and highlights we came across this weekend.
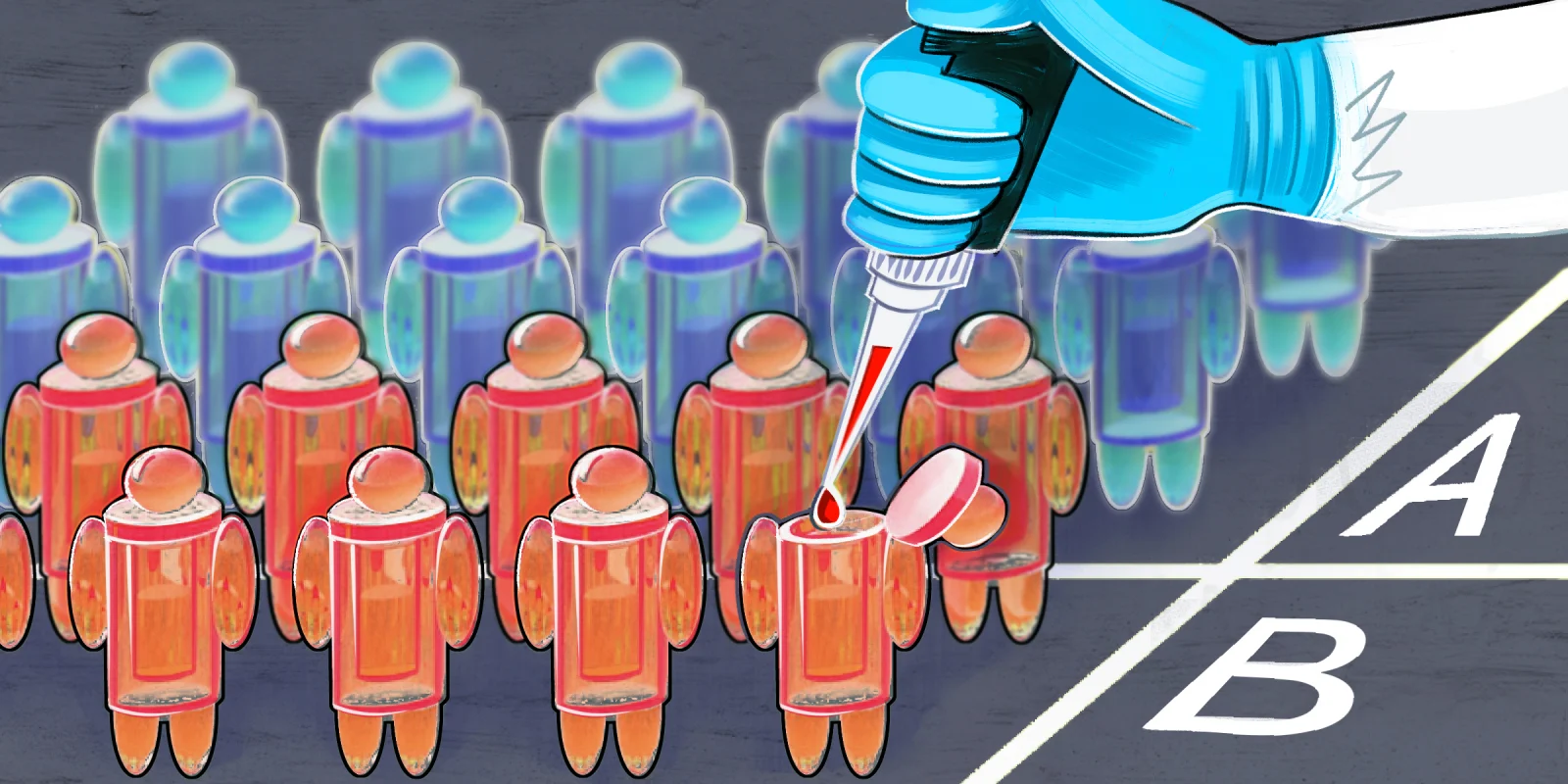
A headline of the AHA conference centered around the SELECT study presented on November 12, in a late-breaking science session. This highly anticipated study addressed the role of pharmacological therapy using semaglutide (Ozempic ®), a glucagon-like peptide (GLP-1) receptor agonist, in non-diabetic obese patients with established cardiovascular disease (defined as a prior myocardial infarction, stroke, or peripheral arterial disease) for secondary prevention of cardiovascular events. It was simultaneously published in the New England Journal of Medicine. Given the prevalence of obesity and the increasing use of these drugs, these findings are sure to create some hype. Briefly, the study showed a nearly 20% relative risk reduction in CV events, including death at 3 years. The absolute risk reduction was more modest at 1.5%. From this we can calculate a number needed to treat (NNT) of 67. In other words, we would need to treat 67 obese people for 3 years with the drug to save 1 event. Given the expensive nature of the drugs, a big question looms. Is it worth the cost? These drugs cost around $1,000-$1,300 per month. Therefore, out-of-pocket costs would equate to an estimated $12,000 or more per year, and $36,000 over the 3 years to treat 1 patient. Based on the NNT analysis, this equates to > $2.4 million, (the cost to treat 67 patients over 3 years) to prevent 1 event at 3 years. Further, these events are not driven by mortality, but are mainly driven by the reduction in non-fatal heart attacks. Further subgroup analysis didn’t show there was a difference based on baseline BMI. There was also a significant reduction in the percentage of patients progressing to diabetes mellitus over 3 years, which may also explain some of the benefit in ASCVD risk reduction.
The SELECT trial leaves us with questions as we decide how to apply these results. Is the cost worth it from a patient and societal standpoint? I’m sure we will see cost effectiveness analysis in the near future. We will need to decide where this fits in our armamentarium, and how to use these drugs strategically and judiciously. Is this a drug effect, or strictly related to benefits of weight loss? Does this commit patients to lifelong therapy, and if not how and when do we deescalate these drugs?
While the SELECT trial was a highlight of the conference focusing on secondary prevention, many sessions focused on caring for the sickest of the sick in the cardiac ICU. Moving the Needle in Cardiogenic Shock was a session led by leaders in the field. While the results of the highly anticipated DanGer trial were not yet available to be discussed, prior large trials on mechanical circulatory support (MCS) were reviewed. Despite the significant advances in medicine and technology, mortality rates with cardiogenic shock remain high and studies have persistently failed to demonstrate an improvement with MCS. However, as cardiologists and intensivists, many of us continue to use these devices, because despite the data, we believe there is some benefit to them. Numerous questions remain about these devices and we all hope our patient will defy the odds and recover with MCS.
An important topic that was discussed during this session that also relates to the SELECT study presentation focused on healthcare disparities. It was highlighted that black and female patients are less likely to receive MCS and have worse outcomes compared to their Caucasian and male counterparts. Although we have not demonstrated a benefit with MCS in studies, as mentioned above, many still believe in them yet we fail to use them equitably. Some of this may be attributed to differences in urban vs rural locations however the possibility of biases or discrimination remains. The benefit of Semaglutide has been proven, however given the cost, who will it be accessible to? We look forward to seeing how the demographics of patients with MCS changes over the upcoming years and similarly the clinical impact of Semaglutide across the nation in improving CV outcomes.
In other exciting developments, the role of artificial intelligence (AI) technology in cardiovascular care was highlighted throughout several sessions. Dr. Demilade A. Adedinsewo, MD presented the SPEC-AI trial which showed AI-guided screening using a digital stethoscope detected twice the number of pregnancy-related cardiomyopathies among nearly 1,200 patients in Nigeria. The ARISE trial showed AI-ECG interpretation shortens the time from ECG acquisition to arriving in the catheterization lab by about 9 minutes in ST-segment elevation myocardial infarctions. The SPEECH trial from Israel - a smartphone app leveraging novel speech analysis technology to detect early signs of worsening HF was 71% accurate in detecting HF events about three weeks in advance. The VERVE trial demonstrated CRISPR DNA editing technology with one injection was able to improve LDL-C and PCSK9 levels in patients with heterozygous familial hypercholesterolemia. The trial serves as a first proof-of-concept for in vivo DNA base editing in humans, and may be a harbinger of future strategies for treatment.
One late breaking trial, the DAPA-MI trial demonstrated a significant benefit of dapagliflozin in cardiometabolic outcomes in patients with acute MI and impaired LV systolic function, but without diabetes or chronic HF during the index hospitalization. There was no impact on the composite of cardiovascular death or hospitalization for heart failure compared with placebo. The ORBITA-2 trial was another placebo, sham-controlled trial similar to the prior ORBITA trial presented by Dr. Christopher Rajkumar. ORBITA-2 demonstrated that among patients with stable angina and coronary stenoses causing ischemia on little or no antianginal therapy, there is a benefit for percutaneous coronary intervention (PCI) for angina relief compared to medical therapy.
In addition to the larger presentations, there was also one my favorite parts of the conference, the abstracts and the oral abstract presentations. There were hundreds of abstracts highlighting the work of early career researchers, clinicians, and trainees. The abstract submissions spanned the full breath of cardiovascular medicine from basic science to epidemiology and clinical care. In addition, simulation sessions helped early trainees gain experience with various procedural techniques and devices. There was a multitude of opportunities for attendees to engage with colleagues, faculty, and trainees. Overall the weekend in Philadelphia was filled with science, and a stimulating look to the future of cardiovascular medicine.
Dr. Alfonso has no conflicts of interest to report.
Illustration by April Brust