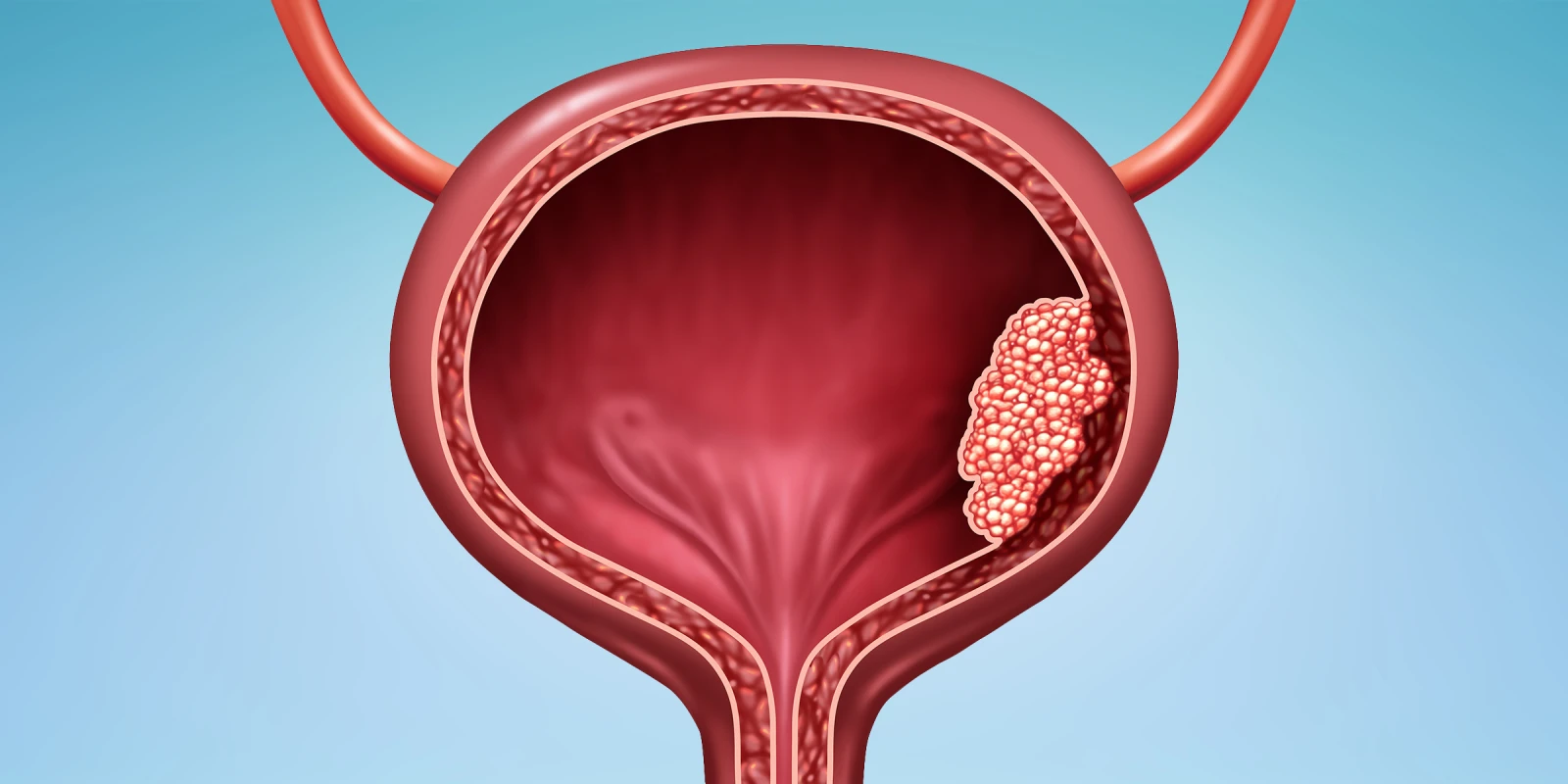
Reasons behind and ways to overcome drug shortages for urothelial carcinoma were addressed at the ASCO GU Symposium.
Elizabeth Guancial, MD, discussed her community practice’s approach to platinum shortages in the US during 2023. They included rounding down doses, switching from carboplatin to cisplatin until the latter became unavailable, temporary restriction to patients with curative-intent treatment, and offering enfortumab vedotin plus pembrolizumab to patients with locally advanced or metastatic bladder cancer before FDA approval.
The impact of these shortages included anxiety for patients, caregivers, and the medical team, treatment delays, dose disruption, increased healthcare workload and costs, and ethical dilemmas. The impact of therapy delay on outcomes is unknown.
Dr. Guancial said that given the complexity of the state of drug production, “this is something we will face again in the future. The sooner practices are notified about potential shortages, the sooner we can implement conservation strategies which were effective in our practice.”
Rick Bangs, a bladder cancer survivor and patient advocate, discussed the roles of patient advocacy in addressing drug shortages. These include educating patients on shortages and navigating them, providing discussion points to address with stakeholders, motivating suppliers, supporting clinical trials to protect standard of care agents in control arms and to test alternatives, and partnering with other affected advocacy groups.
Mr. Banks decried the lack of bipartisan support for legislation to build protections against shortages and remove import restrictions during shortages. Guideline revisions are also important because payers use them for reimbursement of alternative treatments.
Chana Weinstock, MD, from the FDA, said shortages most often occur in older, off-patent drugs with low profit margins, particularly sterile injectables and biologics. The FDA can require manufacturers to report supply disruptions, but cannot require companies to make a drug or more of a drug.
The FDA approved only one strain of BCG, TICE. It has changed its definition of “prior BCG” for trial enrollment, but considers all substrains to be different genetically, molecularly, biochemically, and immunologically, requiring trials for approval.
The FDA expedited review of a new carboplatin generic, encouraged companies with discontinued products to bring them back, and implemented temporary importation of cisplatin related to the shortage.
Joshua Meeks, MD, discussed therapies to address the BCG shortage in bladder cancer. The SWOG1602 trial is comparing the Tokyo-172 strain to TICE, with results expected the end of 2024. BLC3002 is evaluating intravesical gemcitabine+docetaxel with and without a PD-1 agent versus the Danish strain of BCG, given the shortage of TICE, to run the trial. EA8212 BRIDGE is also comparing intravesical gemcitabine+docetaxel with BCG. POTOMAC compares BCG ± durvalumab, including whether durvalumab can replace BCG maintenance. KEYNOTE-676 compares BCG ± pembrolizumab, and is also addressing whether maintenance pembrolizumab can replace some BCG. An intravesical device that releases erdafitinib is in trial for those with FGFR mutations.
Dr. Weinstock quoted Dr. Richard Pazdur, head of the Oncology Center of Excellence, who said, “Ultimately, the FDA cannot solve this problem alone. A shortage is the end result of a failure in the market."
Dr. Lederman has no conflicts of interest to report.
Image by Lightspring / Shutterstock