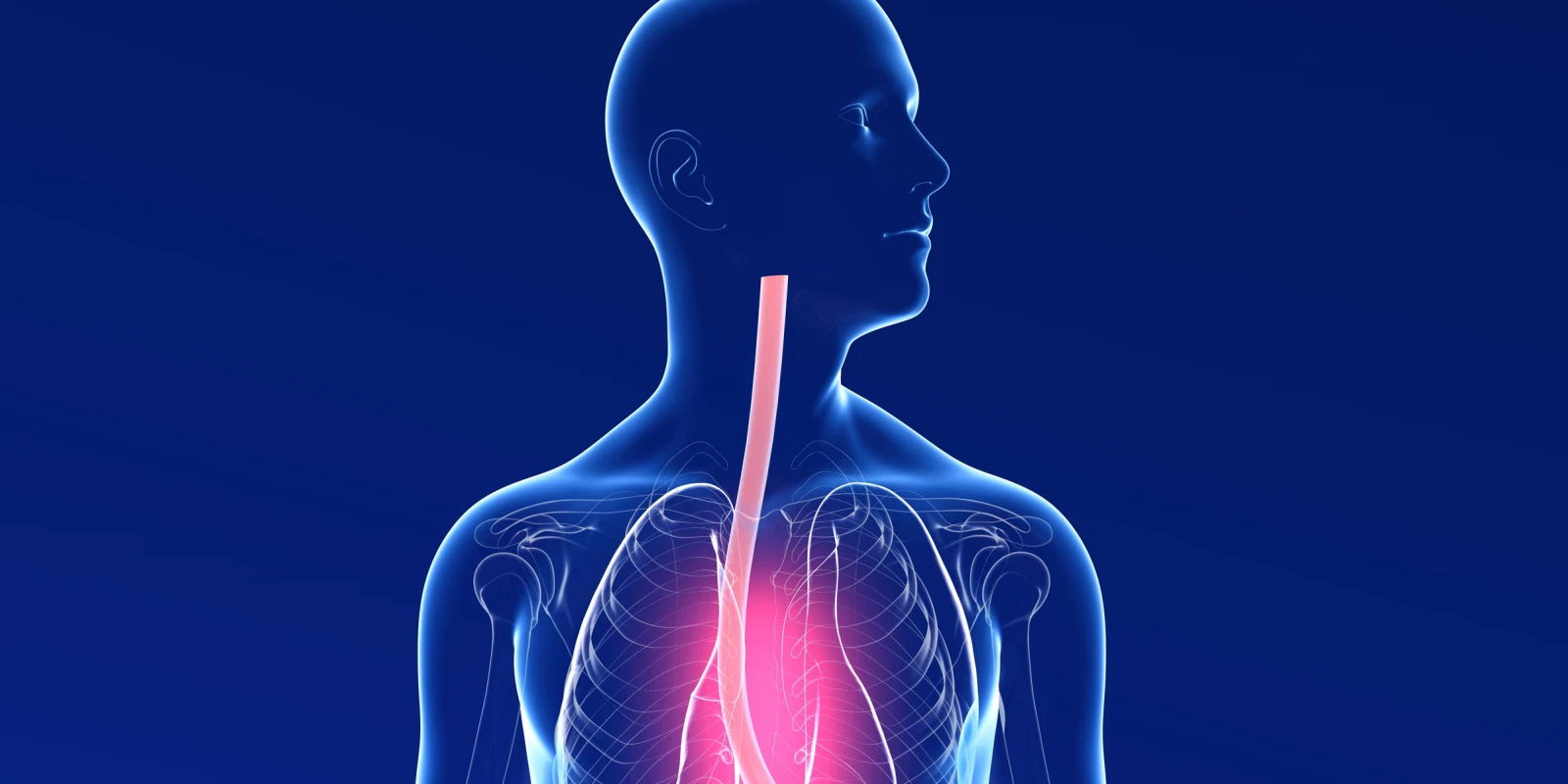
Although every indication has Eosinophilic Esophagitis (EoE) increasing in most areas of the world, especially in first world nations, the term EoE is inaccurate. Using the concept that EoE is more equivalent to “atopic dermatitis of the esophagus,” the true and complex nature of the pathophysiology is more reasonably understood.
At the most recent 2024 Advances and Ongoing Challenges in Eosinophilic Gastrointestinal Disorders: Organized by CIGER/TIGERS at the American Academy of Allergy and Immunology in Washington, D.C., on February 22nd, the immunological complexity of EoE was highlighted, and although the simplicity of eosinophilic infiltration was accepted, it was universally agreed the eosinophil presence is almost an epiphenomenon, and is the natural “end-point” of the complex immune process that occurs prior to the eosinophil infiltration.
The initiation of the pathway ending up as full-blown EoE is unknown. Although, specific genotypes may allow food or antigens, often milk casein, to be presented to the antigen processing cells. This initiation may be enhanced by certain environmental factors, including detergents in household products. The usual relative impermeability of the esophagus epithelium, once breached or injured, allows macro foods antigens (e.g., milk casein) to interact with antigen processing cells and then T cells, both adaptive and innate. This engenders specific CD4+ lymphocyte upregulation, both at a local and, surprisingly, systemically level.
The injury at the epithelial level releases thymic stromal lymphopoietin (TSLP) and Interleukin-33 (IL-33), which enhances the production of other TH2 cytokines (via lymphocytes), including Interleukins 4, 5 and 13 (IL-4, IL-5 and IL-13). B-cells can be activated to produce specific IgE, although not always, and epithelial genes are upregulated, especially Eotaxin 3. These actions are the signals for the attraction of circulating eosinophils to infiltrate, and since many EoE patients are atopic anyway, eosinophils are readily available.
The intense immunologically generated pathway of the adaptive immune system also upregulates innate immune cells,, including ILC-2 cells and mast cells, which are increased in EoE.
Accompanying these immunological aberrations in EoE is a change in the microbiome of the epithelium, possibly allowing for an additional epithelial injury pathway. And very importantly, the neural pathways are dysregulated, likely resulting in pain, dysphagia, and generalized abdominal pain signaling.
Potential immunomodulation of these complex immune processes might include vitamin D supplementation, which decreases IL-13 production, while prebiotics or a high-fiber diet can assist with microbiome realignment.
A very recent pharmacological update has FDA approval of oral viscous budesonide for limited periods, while the anti-IL-4/IL-13 monoclonal antibody Dupilumab has been approved to age one year, with a 15-kilogram limit.
With the genesis of EoE being so immunologically complex, the end-point used in clinical practice of the reduction of eosinophils is critical and measurable, but the prelude immunological processes are becoming prime targets for intervention or even prevention.
With EoE having more understandable complexity, is it time for a new eponym?
Dr. Hopp has no conflicts of interest to report.
Image by Pepe Gallardo / Shutterstock