In recent years we have heard about the “magical” medications that help with weight loss. This medication class is the glucagon-like peptide-1 (GLP-1) receptor agonists. I have seen commercials on YouTube endorsing 30lbs of weight loss in 30 days, read an article in Women’s Health about addiction treatment potential, and read studies showing beneficial cardiovascular (SELECT trial, LEADER trial, REWIND trial) and renal outcomes . This class of medications, however is not new, it has been FDA approved for use since 2005. For the last couple of years there have been numerous news reports about members of this medication class taking turns being in short supply for one reason or another. Social media influencers and celebrities have been blamed for shortages. Meanwhile, insurance providers have become stricter about who can obtain these medications where they are covered for type 2 diabetes only if prescribed as the trade name for diabetes but if prescribing for obesity coverage is less certain and dependent on the company’s recognition of obesity as a medical condition. Navigation of the health system to obtain these medications even became the subject of a recent South Park special.
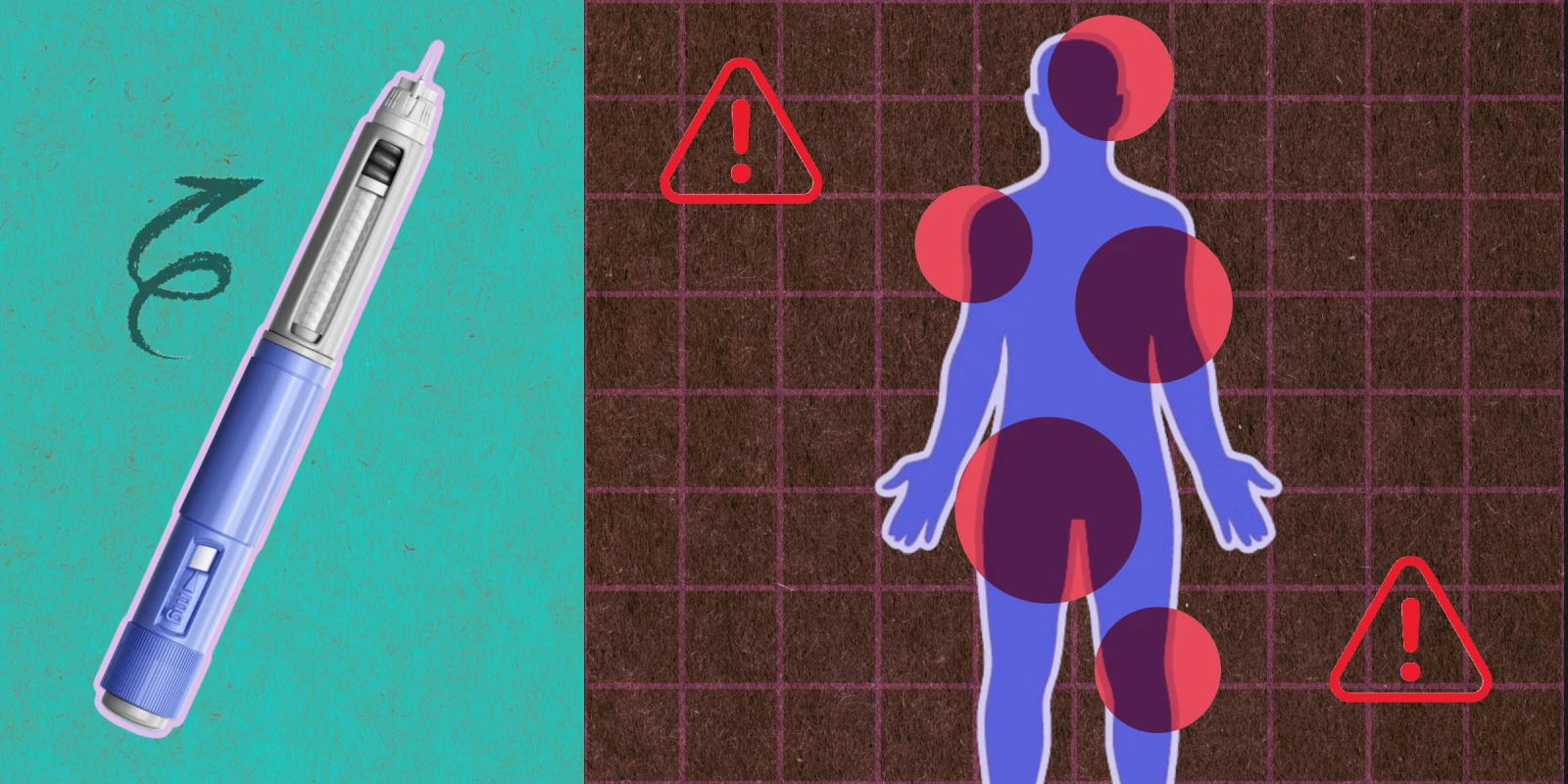
As more people have tried these medications, there have been more reports of side effects that have led to hospitalizations reported in the media. Attending the ADA, I wanted to see what the experts had to say about the side effects of these medications. How does this align with the medical literature and what I have seen in my 12 years of prescribing these medications for people with diabetes? The main session that discussed this was entitled “Severe GLP-1 Side Effects Obesity Treatment--Fact or Fiction?”. Dr. Daniel Drucker led the session with a figure from his recent publication in Diabetes Care that showed established adverse effects, possible adverse effects, and doubtful adverse effects.
Established adverse effects included nausea, vomiting, diarrhea, constipation, gallbladder disease, acute kidney injury and reduced bowel motility. These are well established and each trial that you read where a GLP-1 is studied, alone or in combination with another incretin, you will find the first 4 adverse effects as the most common. Most of these gastrointestinal side effects are mediated through the central nervous system. Acute kidney injury is attributed to decreased hunger and thirst drive leading to decreased fluid intake which contributes to this.
Possible adverse effects included neuropsychiatric events and sarcopenia. Dr. Drucker commented that sarcopenic obesity has not been included in trials and increasing protein and resistance exercise might help. Dr. John-Michael Gambill discussed more of the neuropsychiatric implications focusing mostly on suicide risk. Case reports in 2023 reported more suicidal ideation with semaglutide. He cites that obesity and diabetes increase the risk of suicide, too. There is limited data from randomized control trials and people with severe psychiatric illnesses are excluded from these trials. Small trials have shown neuropsychiatric benefits to depression with GLP-1. More studies are needed.
Doubtful adverse effects included pancreatitis, pancreatic cancer, thyroid cancer, and retinopathy. Dr. Drucker reviewed the evidence about pancreatitis. He notes that in humans GLP-1 reduces pancreatic inflammation and rarely increases amylase and lipase to more than the 3- fold increase which is needed to meet diagnostic criteria for pancreatitis. It has been shown that exenatide stimulates meal time amylase secretion. With respect to pancreatic cancer he shared evidence (1, 2) to indicate that exenatide has decreased growth of tumors. Other types of cancer, colorectal and hepatocellular have also been shown to be reduced. Dr. Elizabeth Pearce discussed thyroid cancer risk and GLP-1. She showed the black box warning in the US regarding the risk of medullary thyroid cancer (MTC) and multiple endocrine neoplasia 2a and 2b. Despite the length of time that these medications have been available and the increase in thyroid cancer diagnosis increasing, mortality rates are flat. MTC being rare at 1-2 % of thyroid cancers, studies need to be much larger to find this risk.
Another topic that was mentioned in this session were “Ozempic babies”. There have been recent reports of more women becoming pregnant while on these medications. In another session at ADA titled, “Myth Busters on “Category X” Drugs in Pregnancy—Evidence for Stopping Commonly Used Medications during Pregnancy, Preconception, and Lactation”, Dr. Maisa Feghali discussed GLP-1 use in women of child-bearing age and the current data available about their use in pregnancy. Increasing data are showing that weight loss with these medications has increased fertility. Exenatide has the most data and doesn’t appear to cross the placenta. The main concern with stopping GLP-1 in pregnancy was increased weight gain and the risk the hyperglycemia leading to more potential for birth defects than the medications themselves. Most doctors, however, are still not likely to keep their pregnant patients on this class of medications due to a lack of data.
Dr. Tchang rounded out the Facts and Fiction Symposium by sharing practical applications for real world use. Ask about personal and family history of thyroid cancer generically, ask about personal history of pancreatitis. Mitigate most common GI side effects with starting low doses, escalating the dose slowly if needed, utilize anti-emetics and prokinetics as needed, eat fiber, maintain hydration, consider coordinating with psychiatrist/therapist, and anticipate need for insulin adjustment to prevent hypoglycemia.
GLP-1s have changed the practice of medicine for both the treatment of type 2 diabetes and obesity. They will change the options for cardiac and renal conditions. The mainstream news continues to report upon them. Patients will ask for them by name. Discussing the risks and benefits with the individual is key given the available evidence. Reports of compounded medications, counterfeit medication and overdoses as a result. Last year calls to poison control were up 1500% . The side effects of these forms are not known in counterfeit and compounded GLP-1s. These sessions discussed studies conducted with FDA approved formulations. Despite the facts, the fiction and the myths, the evolving field of incretin therapies will be fascinating to follow in the coming years.
Dr. Healy has no conflicts of interest to report.
Collage by Diana Connolly