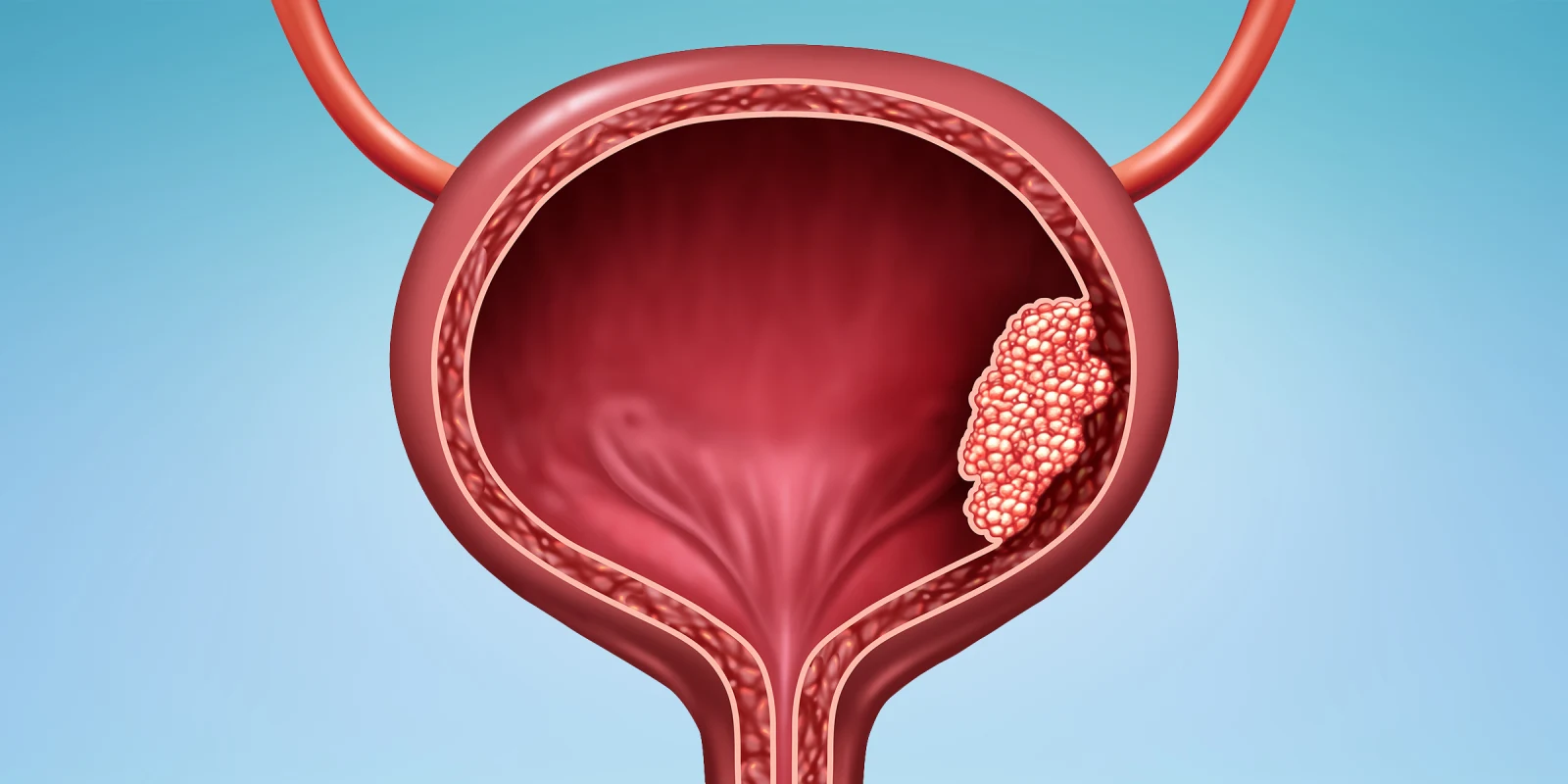
While there were no practice-changing bladder urothelial carcinoma (UC) data, the 2024 ASCO annual conference yielded practice-informing advances. First-line therapy of metastatic UC (mUC) was recently altered by the remarkable results of the phase III EV302 trial comparing Enfortumab Vedotin (EV)+pembrolizumab versus platinum-based chemo in cisplatin-eligible and ineligible patients. EV+pembrolizumab nearly doubled the median progression-free survival (PFS) and overall survival (OS), the dual co-primary endpoints. The confirmed objective response rate (ORR) improved to 68% versus 44%, while complete response (CR) rate also improved to 29.1% versus 12.5%. The major toxicities of EV+pembrolizumab are neuropathy, skin rash, and hyperglycemia. These results are considered transformative and established EV+pembrolizumab as standard first-line therapy for most patients fit for combination therapy. At ASCO 2024, quality of life (QOL) from the EV302 trial was reported based on patient-reported outcomes. EV+pembrolizumab was not associated with detriment to QOL and functioning, which further supports the value of this regimen. Those with moderate to severe pain demonstrated improvements in QOL.
The other recent data that altered first-line therapy was the CHECKMATE901 phase III trial, which compared gemcitabine-cisplatin (GC) versus GC+nivolumab. Both co-primary endpoints, OS and PFS, were better with GC+nivolumab. The ORR was 57.6% versus 43.1%, and CR rate was 21.7% versus 8%. The median duration of CR was remarkably 37.1 months with GC+nivolumab and 13.2 months with GC alone. The GC+nivolumab regimen is characterized by a finite duration of chemotherapy (up to six cycles), which may confer toxicity advantages. Furthermore, the historical track record of cisplatin-based combination consists of cures in a small fraction of patients, which is reinforced by the extremely durable CRs in approximately 22% of patients receiving GC+nivolumab. Thus, there is an impetus to identify predictive biomarkers for CR when employing GC+nivolumab. At ASCO 2024, an oral presentation reported a post hoc analysis to characterize the patient who exhibited CR, with further analysis of patients with lymph node (LN)-only mUC (~18% of patients), who were enriched for CR. Of all 608 randomized patients, 54 treated with NIVO+GC and 56 treated with GC had LN-only mUC. In this subgroup, the ORR and CR rate was 81.5% and 63.0% versus 64.3% and 33.9% for GC+nivolumab and GC, respectively. The median OS in LN-only patients was 46.3 months with GC+nivolumab versus 24.9 with GC, and median PFS was 30.5 months with GC+nivolumab versus 8.8 months with GC. GC+nivolumab generated deep responses and treatment-free intervals in 41% of GC+nivolumab patients with a fixed duration of chemotherapy (up to six cycles) and nivolumab (up to two years). These results provide additional support for GC+nivolumab as a first-line treatment option for selected patients.
An oral presentation reported an analysis of the association between EV plasma exposure and safety and efficacy outcomes. The study included patients in three trials: EV-101 (EV monotherapy 0.75, 1.0, and 1.25 mg/kg on days 1, 8, and 15 of a 28-day cycle [3Q4W]), EV-201, and EV-301 (EV monotherapy 1.25 mg/kg 3Q4W). Dose modifications were common, including dose reductions to 1.0 mg/kg (EV-201 42.1%; EV-301 35.1%) and 0.75 mg/kg (EV-201 13.6%; EV-301 11.1%). EV showed consistent improvement in median PFS and OS versus chemotherapy across all exposures, including those who underwent dose modifications. Greater initial EV exposure in the first two cycles was associated with a higher ORR and was consistent with dose-response. Lower EV exposure was associated with lower risk of EV-related Grade ≥3 toxicities including rash, Grade ≥2 peripheral neuropathy, and Grade ≥3 hyperglycemia. Thus, while the starting dose of 1.25 mg/kg 3Q4W resulted in EV exposure that maximized likelihood of response, dose modifications are effective for managing EV-related AEs and were accompanied by continuing benefit.
Trastuzumab Deruxtecan (T-DXd) received accelerated approval across pre-treated solid tumors with Her2 IHC 3+ expression in the U.S. on April 5, 2024, based on the Destiny PanTumor-02 trial. In 267 pretreated patients with HER2 IHC 2-3+ expressing solid tumors, the ORR was 37.1%. At ASCO 2024, a presentation reported results from the urothelial carcinoma cohort of 41 patients. Most patients had received ≥2 prior regimens. The ORR was 39.0% in the entire group, and was 56.3% in the IHC 3+ group. Drug-related pneumonitis occurred in 9.8% of patients. T-Dxd is a useful addition to the therapeutic armamentarium for the salvage therapy of IHC 3+ (using gastric cancer criteria for Her2 IHC) mUC following prior therapy.
Sacituzumab govitecan (SG) has been used in the clinic based on an accelerated approval by the U.S. FDA in patients with mUC progressing after platinum-based chemotherapy and PD1/L1 inhibitor therapy. On May 30, 2024, a press release from Gilead announced results from the confirmatory Phase 3 TROPiCS-04 trial in locally advanced or mUC, which compared SG versus single-agent chemotherapy following platinum-containing chemotherapy and anti-PD1/L1 therapy. The study unfortunately did not meet the primary endpoint of OS in the intention-to-treat population. However, a numerical improvement in OS favoring SG was observed, and trends in improvement for select pre-specified subgroups and secondary endpoints of PFS and ORR observed. In the overall study population, there was a higher number of deaths due to adverse events with SG, which were primarily observed early in treatment and related to neutropenic complications, including infection. Gilead is further investigating these data and is working to reiterate the importance of granulocyte-colony stimulating factor (G-CSF) for the prevention of neutropenic complications.
The impact of prior perioperative PD1 inhibitor therapy on systemic therapy of mUC remains unclear and warrants study given the availability of adjuvant nivolumab and potential availability of adjuvant pembrolizumab in the future. Indeed, the potential emergence of EV+pembrolizumab and GC+PD1/L1 inhibitors as neoadjuvant therapy will likely again alter the therapeutic landscape of metastatic disease. Given the explosion of new agents, the discovery of predictive biomarkers to inform therapy has become critically important since the delivery of multiple lines of therapy and aggressive multi-agent combinations is likely to reach a ceiling. Real world data are required to shed insights regarding sequencing of therapeutic agents. Clinical trials should be considered a preferred standard of care to improve cure rates and durable survival.
Dr. Sonpavde has received grants from Sanofi, Astrazeneca, Gilead, Helsinn, Lucence, BMS, EMD Serono, Jazz Therapeutics. He has received consulting fees or honorarium from BMS, Genentech, EMD Serono, Merck, Astrazeneca, Sanofi, Seattle Genetics/Astellas, Astrazeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Gilead, Scholar Rock, G1 Therapeutics, Eli Lilly/Loxo Oncology, Infinity Pharmaceuticals, Lucence Health, IMV, Vial, Syapse, Tempus, Ellipses Pharma, PrecisCa, Primum, Syapse, Servier, Merck, Syncorp. He has also received support for participation in review meetings from Mereo. He has received payment for writings from Uptodate, Practice Update, Onviv. He has also received payment for lectures from BIO – INFORMAÇÃO BRASILEIRA DE ONCOLOGIA Ltda, OLE Forum (Mexico), Seagen, Gilead, Natera, Exelixis, Janssen, Bayer, Aveo.
Image by Lightspring / Shutterstock