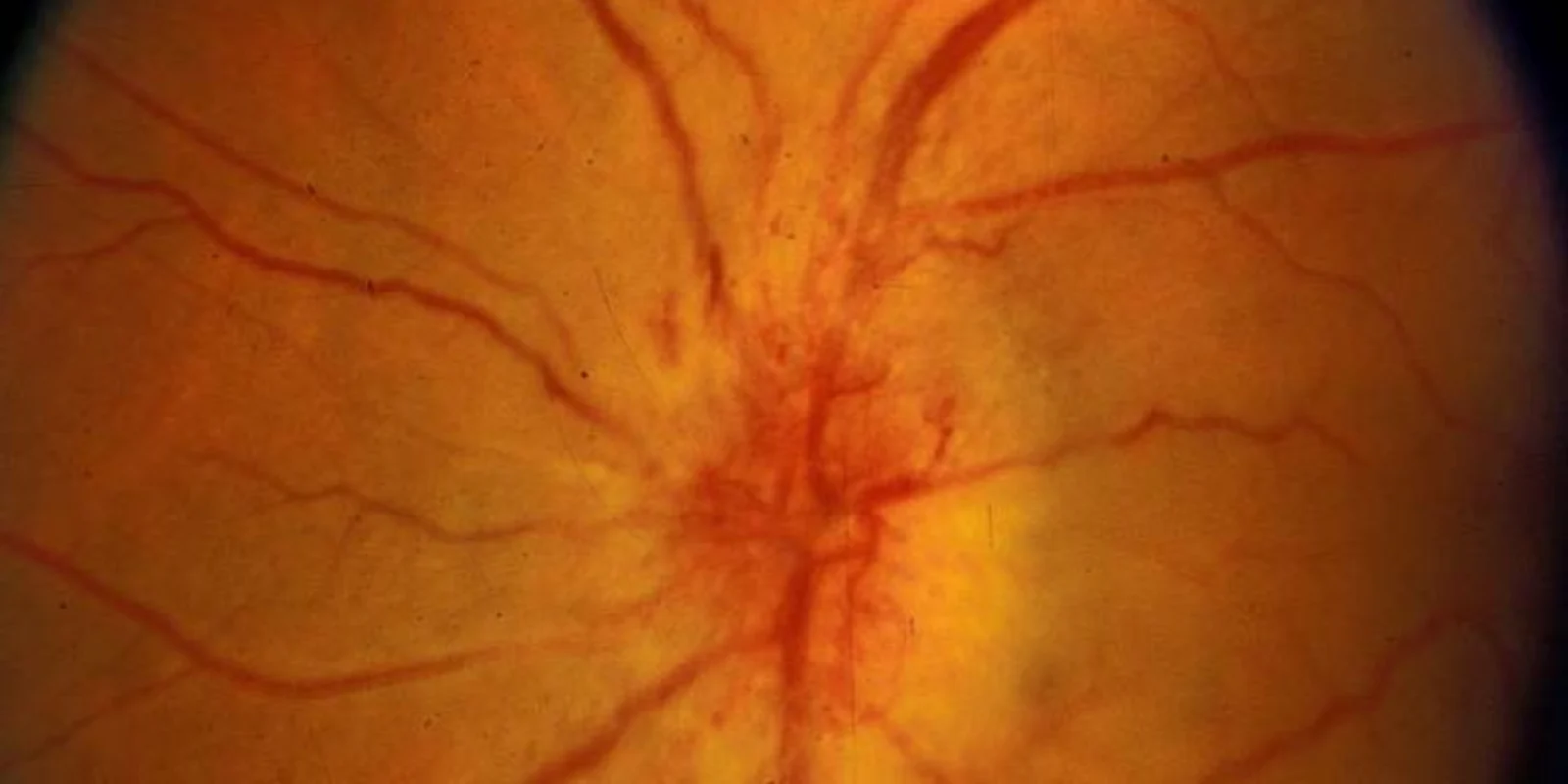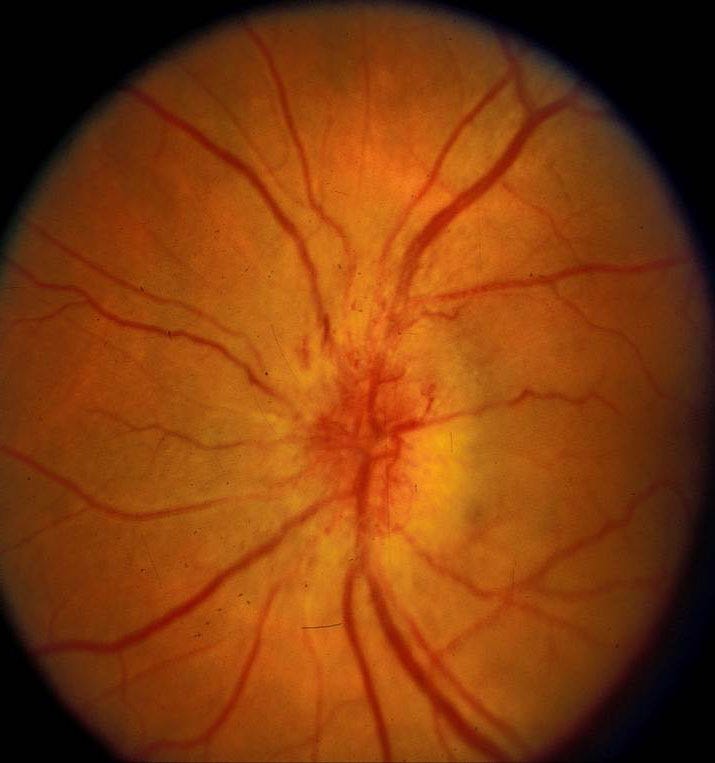
As a practicing neuro-ophthalmologist for over 40 years, I have dealt with many sight-threatening conditions but few are as frustrating for both patients and me as nonarteritic anterior ischemic optic neuropathy (NAION). NAION is the most common cause of optic nerve-related sudden vision loss in the United States, with more than 10,000 cases occurring every year, and is second only to glaucoma in causing optic nerve damage in patients over age 50. NAION can be thought of as a ‘stroke’ of the optic nerve, the nerve that transmits visual images to the brain, and commonly occurs in patients with a congenitally small optic disc (the part of the optic nerve that can be seen with an ophthalmoscope) with a small central depression (the optic cup) as well as underlying systemic vascular risk factors, including obesity, diabetes, and hypertension, that may or may not be known at the time the patient develops the condition. In such patients, visual acuity typically declines to 20/200 or worse and is associated with a significant visual field defect and optic disc swelling, often severe, that gradually resolves over 6–11 weeks, leaving optic atrophy.


Although NAION begins as a unilateral disorder, 15–20% of individuals who develop NAION in one eye experience a similar event in the fellow eye, usually within 5 years of the first event, leading to severe functional and emotional disability and significant economic consequences for the patient and his/her family. Very little is understood concerning the pathophysiology of the disease, and there currently is no medical or surgical treatment that has been proven consistently beneficial. Indeed, ophthalmologists have tried numerous treatments for patients with NAION, ranging from steroids to anti-VEGF agents, all with mixed results. Now, a multicenter phase 2/3 clinical study, for which I am the study director, is underway to try a new neuroprotective drug as a potential treatment.
The drug used in the clinical study is called QPI-1007 (developed by Quark Pharmaceuticals). It is a synthetic small interfering RNA (siRNA) that targets the mRNA of the caspase 2 gene and is designed to inhibit the expression of caspase 2, and stop cell death, via the RNA interference pathway. The clinical study is being performed in a randomized, masked fashion so that neither the patient nor the primary investigator knows what the subject is receiving.
The clinical study, which is Sponsored by Quark Pharmaceuticals and supported by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC), plans to enroll up to 530 subjects and complete the study by July 2019. To date, over 200 patients have been randomized at approximately 90 sites around the world, including over 40 sites in the United States. Inclusion criteria for the clinical study include symptoms for fewer than 14 days, a clinical picture (including visual field defects and abnormalities on optical coherence tomography) consistent with NAION, naïve to any NAION treatment, and no other ocular conditions that could confuse subsequent assessments (e.g., macular degeneration, glaucoma). Subjects must be between 50 and 80 years old. If this study drug proves beneficial in patients with NAION, it could preserve visual function in thousands, perhaps millions of patients, worldwide.
Physicians who have a patient with acute NAION who may qualify for the clinical study or who wish more details of the study should go to www.nordicclinicaltrials.com/naion-doctor-fact-sheet. Public information about NAION and the QRK207 study is available on www.EyeActNow.com or www.clinicaltrials.gov.