*Last updated on 4/10/2020
Click here for the list of trials outside the U.S.
*Data source: www.clinicaltrials.gov
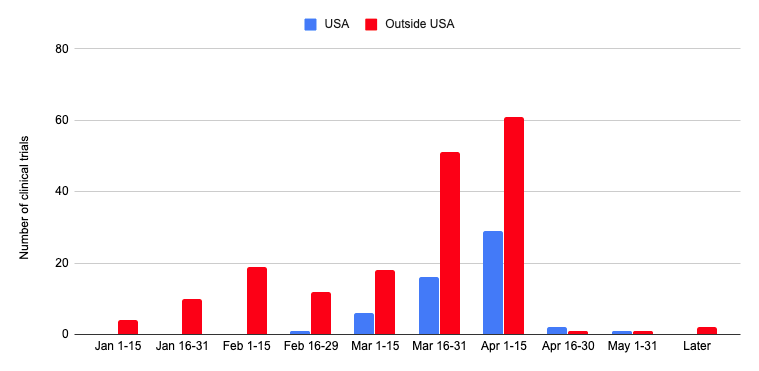
With many asking questions about existing vaccine and/or therapeutic clinical trials, the Doximity Editorial team has compiled a list of relevant and ongoing or planned studies found on clinicaltrials.gov. Above, you will find the number of studies being conducted in the U.S. (blue) and outside the U.S. (red) since the first cluster of COVID-19 cases reported by Chinese officials on December 31, 2019.
*Data source: www.clinicaltrials.gov
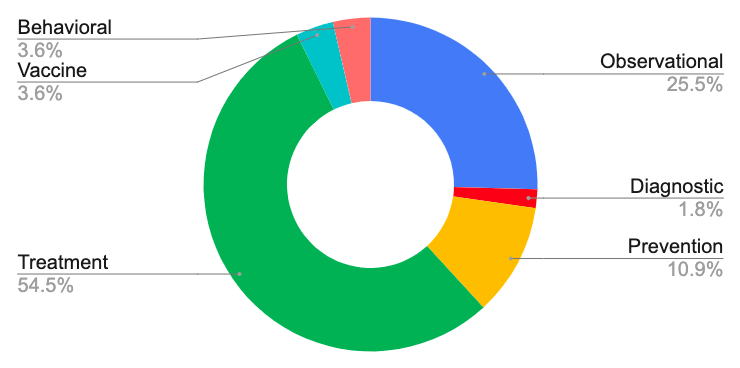
The majority (62.5%) of clinical trials in the U.S. are investigating treatment options for COVID-19 (see graph above). Many of the trials represented are investigating repurposed drugs. Gilead’s remdesivir, an intravenous antiviral treatment, is currently in a phase 3 clinical trial and in recent news, they gave up orphan status which allowed for the earlier release of generic competitors. A phase 3 clinical trial has also been launched testing the effectiveness of hydroxychloroquine, a drug brought to public attention by the President. Use of convalescent plasma collected from individuals who have recovered from COVID-19 as a method of providing viral antibodies to patients has also garnered attention from the FDA. In addition to these high profile drugs, studies are also investigating the therapeutic potential of other agents such as aviptadil, CD24Fc, and stem cell therapy. For information about recommended treatments and access to remdesivir and hydroxychloroquine, check out the CDC’s guide to current therapeutic options for COVID-19.
There are also notable efforts to develop novel therapies and vaccines for COVID-19. For commentary on these investigations worldwide, STAT's guide is a good resource.
For individual reference, we have compiled a list of all clinical trials following this article separated by geographic location. As this health crisis develops, Doximity hopes to be a source of relevant and useful information for those on the front lines.
Thank you,
Doximity Editorial Team
Doximity is working hard to make your clinical lives easier during this pandemic. For more information about how Doximity can help you, click here.
Clinical Trials in the United States
NCT04341935: Effects of DPP4 Inhibition on COVID-19
Study Type: Treatment
Study Phase: 4
Status: Not yet recruiting
Enrollment #: 20
Start Date: 4/30/2020
Primary Completion Date: 6/30/2020
NCT04334967: Hydroxychloroquine in Patients With Newly Diagnosed COVID-19 Compared to Standard of Care
Study Type: Treatment
Study Phase: 4
Status: Enrolling by invitation
Enrollment #: 1250
Start Date: 3/30/2020
Primary Completion Date: 9/30/2021
NCT04340557: Do Angiotensin Receptor Blockers Mitigate Progression to Acute Respiratory Distress Syndrome With SARS-CoV-2 Infection
Study Type: Treatment
Study Phase: 4
Status: Recruiting
Enrollment #: 200
Start Date: 3/27/2020
Primary Completion Date: 10/6/2020
NCT04328467: Pre-exposure Prophylaxis for SARS-Coronavirus-2
Study Type: Prevention
Study Phase: 3
Status: Recruiting
Enrollment #: 3500
Start Date: 4/6/2020
Primary Completion Date: 8/1/2020
NCT04341441: Will Hydroxychloroquine Impede or Prevent COVID-19
Study Type: Prevention
Study Phase: 3
Status: Not yet recruiting
Enrollment #: 3000
Start Date: 4/7/2020
Primary Completion Date: 7/1/2020
NCT04308668: Post-exposure Prophylaxis / Preemptive Therapy for SARS-Coronavirus-2
Study Type: Treatment
Study Phase: 3
Status: Recruiting
Enrollment #: 3000
Start Date: 3/17/2020
Primary Completion Date: 4/21/2020
NCT04280705: Adaptive COVID-19 Treatment Trial (ACTT)
Study Type: Treatment
Study Phase: 3
Status: Recruiting
Enrollment #: 440
Start Date: 2/21/2020
Primary Completion Date: 4/1/2023
NCT04292899: Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Severe Coronavirus Disease (COVID-19)
Study Type: Treatment
Study Phase: 3
Status: Recruiting
Enrollment #: 2400
Start Date: 3/6/2020
Primary Completion Date: 5/1/2020
NCT04292730: Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment
Study Type: Treatment
Study Phase: 3
Status: Recruiting
Enrollment #: 1600
Start Date: 3/15/2020
Primary Completion Date: 5/1/2020
NCT04322682: Colchicine Coronavirus SARS-CoV2 Trial (COLCORONA)
Study Type: Treatment
Study Phase: 3
Status: Recruiting
Enrollment #: 6000
Start Date: 3/23/2020
Primary Completion Date: 9/1/2020
NCT04317040: CD24Fc as a Non-antiviral Immunomodulator in COVID-19 Treatment
Study Type: Treatment
Study Phase: 3
Status: Recruiting
Enrollment #: 230
Start Date: 4/8/2020
Primary Completion Date: 5/1/2021
NCT04341727: Hydroxychloroquine,Hydroxychloroquine,Azithromycin in the Treatment of SARS CoV-2 Infection
Study Type: Treatment
Study Phase: 3
Status: Recruiting
Enrollment #: 500
Start Date: 4/4/2020
Primary Completion Date: 4/1/2021
NCT04326426: ODYSSEY: A Study to Investigate the Efficacy of Tradipitant in Treating Severe or Critical COVID-19 Infection
Study Type: Treatment
Study Phase: 3
Status: Not yet recruiting
Enrollment #: 300
Start Date: 4/1/2020
Primary Completion Date: 8/1/2020
NCT04332991: Outcomes Related to COVID-19 Treated With Hydroxychloroquine Among In-patients With Symptomatic Disease
Study Type: Treatment
Study Phase: 3
Status: Recruiting
Enrollment #: 510
Start Date: 4/2/2020
Primary Completion Date: 4/1/2021
NCT04318444: Hydroxychloroquine Post Exposure Prophylaxis for Coronavirus Disease (COVID-19)
Study Type: Prevention
Study Phase: 2|3
Status: Not yet recruiting
Enrollment #: 1600
Start Date: 3/1/2020
Primary Completion Date: 3/1/2021
NCT04328012: COVID MED Trial - Comparison Of Therapeutics for Hospitalized Patients Infected With SARS-CoV-2
Study Type: Treatment
Study Phase: 2|3
Status: Recruiting
Enrollment #: 4000
Start Date: 4/6/2020
Primary Completion Date: 1/1/2021
NCT04315298: Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19
Study Type: Treatment
Study Phase: 2|3
Status: Recruiting
Enrollment #: 400
Start Date: 3/18/2020
Primary Completion Date: 3/9/2021
NCT04312243: NO Prevention of COVID-19 for Healthcare Providers
Study Type: Prevention
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 470
Start Date: 4/2/2020
Primary Completion Date: 3/20/2021
NCT04333732: CROWN CORONATION: Chloroquine RepurpOsing to healthWorkers for Novel CORONAvirus mitigaTION
Study Type: Prevention
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 55000
Start Date: 4/1/2020
Primary Completion Date: 2/1/2021
NCT04299152: Stem Cell Educator Therapy Treat the Viral Inflammation Caused by Severe Acute Respiratory Syndrome Coronavirus 2
Study Type: Treatment
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 20
Start Date: 4/10/2020
Primary Completion Date: 10/9/2020
NCT04312009: Losartan for Patients With COVID-19 Requiring Hospitalization
Study Type: Treatment
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 200
Start Date: 4/2/2020
Primary Completion Date: 4/1/2021
NCT04311177: Losartan for Patients With COVID-19 Not Requiring Hospitalization
Study Type: Treatment
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 516
Start Date: 4/2/2020
Primary Completion Date: 4/1/2021
NCT04311697: Intravenous Aviptadil for COVID-19 Associated Acute Respiratory Distress
Study Type: Treatment
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 120
Start Date: 4/1/2020
Primary Completion Date: 8/1/2020
NCT04323800: Efficacy and Safety Human Coronavirus Immune Plasma (HCIP) vs. Control (SARS-CoV-2 Non-immune Plasma) Among Adults Exposed to COVID-19
Study Type: Treatment
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 150
Start Date: 5/1/2020
Primary Completion Date: 12/31/2022
NCT04342169: University of Utah COVID-19 Hydrochloroquine Trial
Study Type: Treatment
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 400
Start Date: 4/14/2020
Primary Completion Date: 4/1/2022
NCT04338074: TXA and Corona Virus 2019 (COVID19) in Outpatients
Study Type: Treatment
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 100
Start Date: 4/15/2020
Primary Completion Date: 10/15/2020
NCT04338126: Tranexamic Acid (TXA) and Corona Virus 2019 (COVID19) in Inpatients
Study Type: Treatment
Study Phase: 2
Status: Not yet recruiting
Enrollment #: 60
Start Date: 4/15/2020
Primary Completion Date: 10/15/2020
NCT04341116: Study of TJ003234 (Anti-GM-CSF Monoclonal Antibody) in Subjects With Severe Coronavirus Disease 2019 (COVID-19)
Study Type: Treatment
Study Phase: 1|2
Status: Not yet recruiting
Enrollment #: 144
Start Date: 4/1/2020
Primary Completion Date: 9/1/2020
NCT04328961: Hydroxychloroquine for COVID-19 PEP
Study Type: Prevention
Study Phase: 1
Status: Not yet recruiting
Enrollment #: 2000
Start Date: 3/1/2020
Primary Completion Date: 9/30/2020
NCT04340050: COVID-19 Convalescent Plasma
Study Type: Treatment
Study Phase: 1
Status: Not yet recruiting
Enrollment #: 10
Start Date: 4/30/2020
Primary Completion Date: 12/31/2020
NCT04333654: Hydroxychloroquine in Outpatient Adults With COVID-19
Study Type: Treatment
Study Phase: 1
Status: Recruiting
Enrollment #: 210
Start Date: 3/31/2020
Primary Completion Date: 5/1/2020
NCT04333251: Study Testing Convalescent Plasma vs Best Supportive Care
Study Type: Treatment
Study Phase: 1
Status: Not yet recruiting
Enrollment #: 115
Start Date: 4/1/2020
Primary Completion Date: 12/31/2022
NCT04326036: Use of cSVF Via IV Deployment for Residual Lung Damage After Symptomatic COVID-19 Infection
Study Type: Treatment
Study Phase: 1
Status: Enrolling by invitation
Enrollment #: 10
Start Date: 3/25/2020
Primary Completion Date: 11/1/2021
NCT04283461: Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection
Study Type: Vaccine
Study Phase: 1
Status: Recruiting
Enrollment #: 45
Start Date: 3/3/2020
Primary Completion Date: 6/1/2021
NCT04336410: Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers
Study Type: Vaccine
Study Phase: 1
Status: Recruiting
Enrollment #: 40
Start Date: 4/3/2020
Primary Completion Date: 11/1/2020
NCT04339998: Assessment of Exam Findings in Coronavirus Disease 2019 (COVID-19) With Point-of-Care Ultrasonography (POCUS)
Study Type: Diagnostic
Study Phase: Not provided
Status: Not yet recruiting
Enrollment #: 500
Start Date: 4/15/2020
Primary Completion Date: 10/1/2020
NCT04331886: An Observational Study of Patients With Coronavirus Disease 2019
Study Type: Observational
Study Phase: Not provided
Status: Not yet recruiting
Enrollment #: 5000
Start Date: 4/1/2020
Primary Completion Date: 3/1/2021
NCT04321811: Behavior, Environment And Treatments for Covid-19
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 100000
Start Date: 3/21/2020
Primary Completion Date: 3/20/2021
NCT04323839: COVID-19 PRIORITY (Pregnancy CoRonavIrus Outcomes RegIsTrY)
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 1000
Start Date: 3/20/2020
Primary Completion Date: 3/31/2024
NCT04330521: Impact of the Coronavirus (COVID-19) on Patients With Cancer
Study Type: Observational
Study Phase: Not provided
Status: Not yet recruiting
Enrollment #: 50
Start Date: 4/1/2020
Primary Completion Date: 5/1/2022
NCT04339387: COVID-19 Risk Stratification
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 1500
Start Date: 3/1/2020
Primary Completion Date: 4/15/2020
NCT04336215: Rutgers COVID-19 Cohort Study
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 750
Start Date: 3/28/2020
Primary Completion Date: 9/1/2020
NCT04323787: Viral Infection and Respiratory Illness Universal Study[VIRUS]: COVID19 Registry
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 50000
Start Date: 3/30/2020
Primary Completion Date: 4/30/2021
NCT04342195: Acquiring Convalescent Specimens for COVID-19 Antibodies
Study Type: Observational
Study Phase: Not provided
Status: Not yet recruiting
Enrollment #: 12
Start Date: 4/1/2020
Primary Completion Date: 3/1/2021
NCT04335630: Cardiovascular Manifestations of COVID-19
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 500
Start Date: 3/30/2020
Primary Completion Date: 3/1/2021
NCT04327804: A Longitudinal Study of SARS-CoV-2 Positive Patients Testing Nasal Swabs and Collecting Blood Samples for Research
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 120
Start Date: 3/25/2020
Primary Completion Date: 4/3/2020
NCT04320511: Beaumont Quantitative Lung Function Imaging to Characterize Patients With SARS-COV 2
Study Type: Observational
Study Phase: Not provided
Status: Not yet recruiting
Enrollment #: 25
Start Date: 4/1/2020
Primary Completion Date: 3/1/2021
NCT04326309: Audio Data Collection for Identification and Classification of Coughing
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 1000
Start Date: 3/25/2020
Primary Completion Date: 9/25/2022
NCT04320862: Pandemic Response Network
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 200000
Start Date: 4/3/2020
Primary Completion Date: 12/31/2021
NCT04339790: Mental Health Impact of COVID-19 Pandemic on NIMH Research Participants and Volunteers
Study Type: Observational
Study Phase: Not provided
Status: Recruiting
Enrollment #: 5000
Start Date: 4/15/2020
Primary Completion Date: 3/1/2022
NCT04329533: Effects of Using Mobile App on Perceived Stress During Coronavirus ("COVID 19") Pandemic
Study Type: Behavioral
Study Phase: Not Applicable
Status: Not yet recruiting
Enrollment #: 150
Start Date: 4/1/2020
Primary Completion Date: 6/30/2020
NCT04329897: Acceptance and Commitment Therapy Delivered by Automated Software Messaging
Study Type: Behavioral
Study Phase: Not Applicable
Status: Enrolling by invitation
Enrollment #: 82
Start Date: 4/5/2020
Primary Completion Date: 5/30/2020
NCT04338009: Elimination or Prolongation of ACE Inhibitors and ARB in Coronavirus Disease 2019
Study Type: Treatment
Study Phase: Not Applicable
Status: Not yet recruiting
Enrollment #: 152
Start Date: 3/31/2020
Primary Completion Date: 12/31/2020
NCT04326452: Treating COVID-19 With a Bidirectional Oxygenation Valve
Study Type: Treatment
Study Phase: Not Applicable
Status: Recruiting
Enrollment #: 15
Start Date: 3/27/2020
Primary Completion Date: 5/1/2020
NCT04325906: Early PP With HFNC Versus HFNC in COVID-19 Induced Moderate to Severe ARDS
Study Type: Treatment
Study Phase: Not Applicable
Status: Not yet recruiting
Enrollment #: 346
Start Date: 4/6/2020
Primary Completion Date: 5/31/2020
Illustration by April Brust
Click here to see more perspectives on COVID-19 from the Doximity network.
Click here for up-to-date news about COVID-19 on Doximity.